Cobalamin
- vitamin B12
- cobalamin
- cyanocobalamin
- vitamin B-12
- 68-19-9
- Create:2010-08-30
- Modify:2025-01-18
 vitamin B12 (preferred).
vitamin B12 (preferred).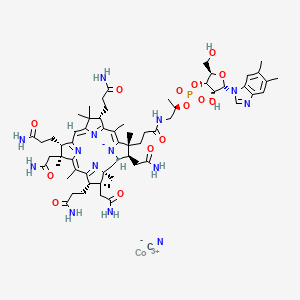
- B 12, Vitamin
- B12, Vitamin
- Cobalamin
- Cobalamins
- Cyanocobalamin
- Eritron
- Vitamin B 12
- Vitamin B12
 vitamin B12 (preferred)
vitamin B12 (preferred)B - Blood and blood forming organs
B03 - Antianemic preparations
B03B - Vitamin b12 and folic acid
B03BA - Vitamin b12 (cyanocobalamin and analogues)
B03BA01 - Cyanocobalamin
Use (kg; approx.) in Germany (2009): >75
Consumption (g per capita; approx.) in Germany (2009): 0.000916
Information on 3 consumer products that contain Cyanocobalamin in the following categories is provided:
• Personal Care
Not Classified
Reported as not meeting GHS hazard criteria by 133 of 134 companies (only 0.7% companies provided GHS information). For more detailed information, please visit ECHA C&L website.
Aggregated GHS information provided per 134 reports by companies from 2 notifications to the ECHA C&L Inventory.
Reported as not meeting GHS hazard criteria per 133 of 134 reports by companies. For more detailed information, please visit ECHA C&L website.
There is 1 notification provided by 1 of 134 reports by companies with hazard statement code(s).
Information may vary between notifications depending on impurities, additives, and other factors. The percentage value in parenthesis indicates the notified classification ratio from companies that provide hazard codes. Only hazard codes with percentage values above 10% are shown.
◉ Summary of Use during Lactation
Vitamin B12 is a normal component of human milk. The recommended daily intake in lactating women is 2.8 mcg and for infants aged 6 months or less is 0.4 mcg. Some authorities recommend 5.5 mcg per day during lactation. Supplementation may be necessary to achieve these recommended daily intakes or to correct a known deficiency. Low doses (1 to 10 mcg) of vitamin B12 found in B complex or prenatal vitamins increase milk levels only slightly. Higher daily doses of 50 to 250 mcg are needed in cases of maternal deficiency. The breastfed infant is not exposed to excessive vitamin B12 in such cases, and their vitamin B12 status should improve if it was previously inadequate.
Poor health outcomes in infants with vitamin B12 deficiency include anemia, abnormal skin and hair development, convulsions, weak muscle tone, failure to thrive, mental developmental delay, and potentially abnormal movements. Well-recognized at risk groups are exclusively breastfed infants of mothers with B12 deficiency due to minimal or no dietary intake of animal products or pernicious anemia caused by a maternal malabsorption of B12. Infant vitamin B12 status can be improved through maternal B12 supplementation during pregnancy and lactation. Deficient mothers who miss the opportunity to supplement during pregnancy should still be encouraged to supplement during early lactation since infant vitamin B12 status correlates with milk vitamin B12 levels in breastfed infants up to 6 months of age. Although there are cases reported of exclusively breastfed infants with vitamin B12 deficiency having biochemical and clinical improvement through adequate maternal supplementation alone, direct supplementation of the infant is recommended when such treatments are available.
◉ Effects in Breastfed Infants
Twelve exclusively breastfed infants between 4 and 11 months of age had biochemical, hematological and clinical findings consistent with vitamin B12 deficiency. Their mothers received a 50 mcg single dose of intramuscular vitamin B12. Within 5 to 8 days after the dose, the infants experienced significantly increased hemoglobin and reticulocyte counts, normoblastic erythropoiesis, improved mental status, regression of abnormal skin pigmentation, and reduction in tremors.
Three hundred sixty-six pregnant women in India received 50 mcg of oral vitamin B12 or placebo capsules once daily beginning during their first trimester of pregnancy and continuing until 6 weeks postpartum. Among 218 infants that underwent neurodevelopment testing at 30 months of age, those born to mothers randomized to vitamin B12 had higher expressive language scores than the placebo group when adjusted for baseline maternal vitamin B12 deficiency. Cognitive, receptive language and motor scores were not different between the two groups. Neurophysiological assessments were then conducted at 6 years of age and there were no differences in the measured brain activity between the two groups.
◉ Effects on Lactation and Breastmilk
Relevant published information was not found as of the revision date.
- Australian Industrial Chemicals Introduction Scheme (AICIS)
- DrugBankLICENSECreative Common's Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/legalcode)https://www.drugbank.ca/legal/terms_of_useCyanocobalaminhttps://www.drugbank.ca/drugs/DB00115
- EPA Chemicals under the TSCAVitamin B12https://www.epa.gov/chemicals-under-tscaEPA TSCA Classificationhttps://www.epa.gov/tsca-inventory
- European Chemicals Agency (ECHA)LICENSEUse of the information, documents and data from the ECHA website is subject to the terms and conditions of this Legal Notice, and subject to other binding limitations provided for under applicable law, the information, documents and data made available on the ECHA website may be reproduced, distributed and/or used, totally or in part, for non-commercial purposes provided that ECHA is acknowledged as the source: "Source: European Chemicals Agency, http://echa.europa.eu/". Such acknowledgement must be included in each copy of the material. ECHA permits and encourages organisations and individuals to create links to the ECHA website under the following cumulative conditions: Links can only be made to webpages that provide a link to the Legal Notice page.https://echa.europa.eu/web/guest/legal-noticeCyanocobalaminhttps://chem.echa.europa.eu/100.000.618Cyanocobalamin (EC: 200-680-0)https://echa.europa.eu/information-on-chemicals/cl-inventory-database/-/discli/details/135322
- Hazardous Substances Data Bank (HSDB)CYANOCOBALAMINhttps://pubchem.ncbi.nlm.nih.gov/source/hsdb/2850
- New Zealand Environmental Protection Authority (EPA)LICENSEThis work is licensed under the Creative Commons Attribution-ShareAlike 4.0 International licence.https://www.epa.govt.nz/about-this-site/general-copyright-statement/
- ClinicalTrials.govLICENSEThe ClinicalTrials.gov data carry an international copyright outside the United States and its Territories or Possessions. Some ClinicalTrials.gov data may be subject to the copyright of third parties; you should consult these entities for any additional terms of use.https://clinicaltrials.gov/ct2/about-site/terms-conditions#Use
- Comparative Toxicogenomics Database (CTD)LICENSEIt is to be used only for research and educational purposes. Any reproduction or use for commercial purpose is prohibited without the prior express written permission of NC State University.http://ctdbase.org/about/legal.jsp
- Consumer Product Information Database (CPID)LICENSECopyright (c) 2024 DeLima Associates. All rights reserved. Unless otherwise indicated, all materials from CPID are copyrighted by DeLima Associates. No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://www.whatsinproducts.com/contents/view/1/6Consumer Products Category Classificationhttps://www.whatsinproducts.com/
- DailyMed
- E. coli Metabolome Database (ECMDB)
- NCI Thesaurus (NCIt)LICENSEUnless otherwise indicated, all text within NCI products is free of copyright and may be reused without our permission. Credit the National Cancer Institute as the source.https://www.cancer.gov/policies/copyright-reuse
- Yeast Metabolome Database (YMDB)Cyanocobalaminhttps://www.ymdb.ca/compounds/YMDB01525
- Drugs and Lactation Database (LactMed)
- Drugs@FDALICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linkingCYANOCOBALAMINhttps://www.accessdata.fda.gov/scripts/cder/daf/
- EU Clinical Trials Register
- FDA Orange BookLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- NORMAN Suspect List ExchangeLICENSEData: CC-BY 4.0; Code (hosted by ECI, LCSB): Artistic-2.0https://creativecommons.org/licenses/by/4.0/NORMAN Suspect List Exchange Classificationhttps://www.norman-network.com/nds/SLE/
- FDA Substances Added to FoodLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Haz-Map, Information on Hazardous Chemicals and Occupational DiseasesLICENSECopyright (c) 2022 Haz-Map(R). All rights reserved. Unless otherwise indicated, all materials from Haz-Map are copyrighted by Haz-Map(R). No part of these materials, either text or image may be used for any purpose other than for personal use. Therefore, reproduction, modification, storage in a retrieval system or retransmission, in any form or by any means, electronic, mechanical or otherwise, for reasons other than personal use, is strictly prohibited without prior written permission.https://haz-map.com/AboutVitamin B12https://haz-map.com/Agents/18656
- Metabolomics Workbench
- National Drug Code (NDC) DirectoryLICENSEUnless otherwise noted, the contents of the FDA website (www.fda.gov), both text and graphics, are not copyrighted. They are in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from FDA. Credit to the U.S. Food and Drug Administration as the source is appreciated but not required.https://www.fda.gov/about-fda/about-website/website-policies#linking
- Natural Product Activity and Species Source (NPASS)
- NIPH Clinical Trials Search of Japan
- NLM RxNorm TerminologyLICENSEThe RxNorm Terminology is created by the National Library of Medicine (NLM) and is in the public domain and may be republished, reprinted and otherwise used freely by anyone without the need to obtain permission from NLM. Credit to the U.S. National Library of Medicine as the source is appreciated but not required. The full RxNorm dataset requires a free license.https://www.nlm.nih.gov/research/umls/rxnorm/docs/termsofservice.htmlvitamin B12https://rxnav.nlm.nih.gov/id/rxnorm/11248
- WHO Anatomical Therapeutic Chemical (ATC) ClassificationLICENSEUse of all or parts of the material requires reference to the WHO Collaborating Centre for Drug Statistics Methodology. Copying and distribution for commercial purposes is not allowed. Changing or manipulating the material is not allowed.https://www.whocc.no/copyright_disclaimer/Cyanocobalaminhttps://www.whocc.no/atc_ddd_index/?code=B03BA01
- WikipediaVitamin B12https://en.wikipedia.org/wiki/Vitamin_B12cyanocobalaminhttps://en.wikipedia.org/wiki/Cyanocobalamin
- Medical Subject Headings (MeSH)LICENSEWorks produced by the U.S. government are not subject to copyright protection in the United States. Any such works found on National Library of Medicine (NLM) Web sites may be freely used or reproduced without permission in the U.S.https://www.nlm.nih.gov/copyright.htmlVitamin B 12https://www.ncbi.nlm.nih.gov/mesh/68014805Vitamin B Complexhttps://www.ncbi.nlm.nih.gov/mesh/68014803
- PubChem
- GHS Classification (UNECE)GHS Classification Treehttp://www.unece.org/trans/danger/publi/ghs/ghs_welcome_e.html
- NCBI
 CID 6713940
CID 6713940 CID 104730 (Cobalt)
CID 104730 (Cobalt) CID 768 (Hydrogen Cyanide)
CID 768 (Hydrogen Cyanide)